Bethlehem, PA – May 14, 2024 – Saladax Biomedical, Inc. (Saladax) is pleased to announce that Health Canada has approved Saladax’s 5-Fluorouracil (My5-FU) Assay for sale in Canada in a rapid six days. This approval recognizes the vital importance of implementing updated guidelines and using 5-Fluorouracil (5-FU) TDM to ensure patients undergoing cancer treatment with this chemotherapy agent get the proper dose.
There is increasing concern with 5-FU treatments worldwide because some patients suffer severe toxicities and even death, which may have been avoided. There is growing frustration in the medical community with oncologists who are reticent to adopt the recommended guidelines for 5-FU TDM.
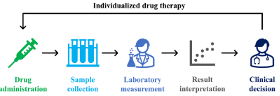
Today, 5-FU infusions are dosed using an antiquated method called “Body Surface Area (BSA).” This method, developed in 1916, is recognized as inadequate. BSA dosing does not account for individual differences between patients, nor does it measure the blood level of the drug in the patient. Multiple studies have demonstrated that most patients are not getting the right dose. Pharmacology guidelines highly recommend that 5-FU levels be measured and adjusted on a patient-by-patient basis. By adjusting the dose to fit the patient, efficacy is improved, and toxicity is minimized.
The Saladax My5-FU blood test, used in multiple countries worldwide, is now approved in Canada, the European Union, China, the United Kingdom, and Israel. The only major country where it is not approved is the United States. The test is remarkably straightforward to implement. Requiring just one blood sample during a chemotherapy cycle, it can easily be run on analyzers already present in clinical laboratories, making levels practical and accessible to healthcare professionals.
“It’s imperative that we move away from the assumption that a patient receives the appropriate 5-FU dose based on a one-size-fits-all approach, which is often not the case. The use of real-time blood levels to guide 5-FU therapy management”, as advocated by Salvatore Salamone, Saladax’s CEO “is a critical step forward. How many more patients will unnecessarily suffer from incorrect dosing before this issue is addressed in the US?”

ABOUT SALADAX BIOMEDICAL, INC.
Headquartered in Bethlehem, PA, Saladax Biomedical, Inc. is a privately held company that develops, manufactures, and markets assays that provide rapid therapeutic drug levels for essential and life-saving medicines prescribed by psychiatrists and oncologists. Since 2007, Saladax’s proprietary technology has been used in clinical laboratories or point-of-care settings to assist clinicians in monitoring and optimizing patient care. Additionally, the company collaborates with leading pharmaceutical companies to develop tests for clinical trials and companion diagnostics. For more information, visit MyCareTests.com